Patient Presentation
An 87-year-old male patient presented with progressively worsening vision over the past year. Despite trialing new eyeglasses, he continued to notice visual decline that interfered with daily functioning. Although advanced in age, he remained highly independent, continuing to drive and serving as the primary caregiver for his ailing wife.
The patient’s ocular history included dry age-related macular degeneration (AMD) with geographic atrophy (GA) OU, posterior chamber intraocular lens OU, and prior YAG capsulotomy OU. He also had a medical history of coronary artery disease, hypertension, hyperlipidemia, and psoriasis. His medications included amlodipine, atorvastatin, clobetasol, and triamterene.
Diagnosis
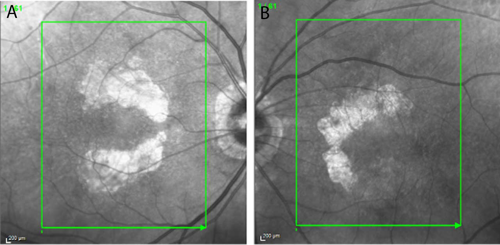
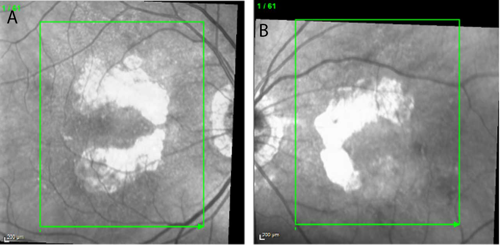
At a recent visit to his optometrist, the patient had been diagnosed with advanced dry AMD with bilateral nonsubfoveal GA. Baseline near-infrared imaging revealed multifocal lesions of 9.11 mm² OD and 5.37 mm² OS. Best-corrected visual acuity (VA) was 20/20 OD and 20/30 OS. Notably, there was no evidence of choroidal neovascularization.
Treatment
Given the patient’s active lifestyle and risk of vision loss progression, I recommended and subsequently initiated bilateral treatment with an intravitreal complement inhibitor on a staggered dosing schedule. The first injection was administered in the left eye in November 2023, followed by injection in both eyes in January 2024. Injections were then continued in both eyes every 6 to 8 weeks to ensure consistent bilateral coverage. My decision to stagger initiation was to ensure that no adverse effects occurred. Thereafter, bilateral administration was able to be maintained.
Outcomes
Over the first year of treatment, VA remained stable. VA in the patient’s right eye fluctuated between 20/20 and 20/25, while that in the left eye ranged between 20/25 and 20/30. Imaging suggested continued enlargement of GA lesions, although the growth rate appeared slower compared with the rapid progression documented in the months prior to the start of treatment (Figures 1 and 2). Equally important, the patient was able to maintain his independence, continued to drive, and remained the caregiver for his wife—outcomes that reflected his own treatment goals.
Discussion
This case reflects the clinical value of an FDA-approved complement inhibitor in the management of GA. The treatment received FDA approval as a therapy for GA secondary to AMD. The agency rendered its decision based on clinical trial data demonstrating that the complement inhibitor slows foveal and subfoveal lesion growth by approximately 20% to 30% over a period of 2 to 3 years.
Additional long-term clinical trial data further demonstrated that sustained treatment yields increasing benefit, with greater reduction in lesion growth observed over time compared with early discontinuation.1,2
In this patient, bilateral nonsubfoveal lesions provided a strong rationale for early intervention. Pretreatment imaging showed significant lesion growth during the 5 months between May and October 2023. After treatment, the lesions continued to grow but the growth rate was likely slowed owing to the intervention.
By initiating treatment while the patient still had strong central VA, the patient was able to maintain his independence and quality of life—outcomes supported by clinical trial evidence that earlier treatment may preserve vision-related function.1,2
Flexible dosing was also an important factor in this patient’s ability to adhere to treatment. Enabling individualized dosing intervals (every 25 to 60 days) has been shown to provide a balance between efficacy and patient burden.1
Conclusion
This case stresses the importance of early initiation of bilateral therapy with complement inhibitor therapy in slowing GA progression. For this highly active elderly patient, leveraging flexible dosing and committing to long-term treatment has been helping to preserve not just his visual acuity, but also his independence in daily living.
References
- Wykoff CC, Holz FG, Chiang A, et al. Pegcetacoplan treatment for geographic atrophy in age-related macular degeneration over 36 months: data from OAKS, DERBY, and GALE. Am J Ophthalmol. 2025;276:350-364. doi:10.1016/j.ajo.2025.04.0164.
- Heier JS, Lad EM, Holz FG, et al. Pegcetacoplan for the treatment of geographic atrophy secondary to age-related macular degeneration (OAKS and DERBY): two multicentre, randomised, double-masked, sham-controlled, phase 3 trials. Lancet. 2023;402(10411):1434-1448. doi:10.1016/S0140-6736(23)01520-9
This editorial content is sponsored by 









