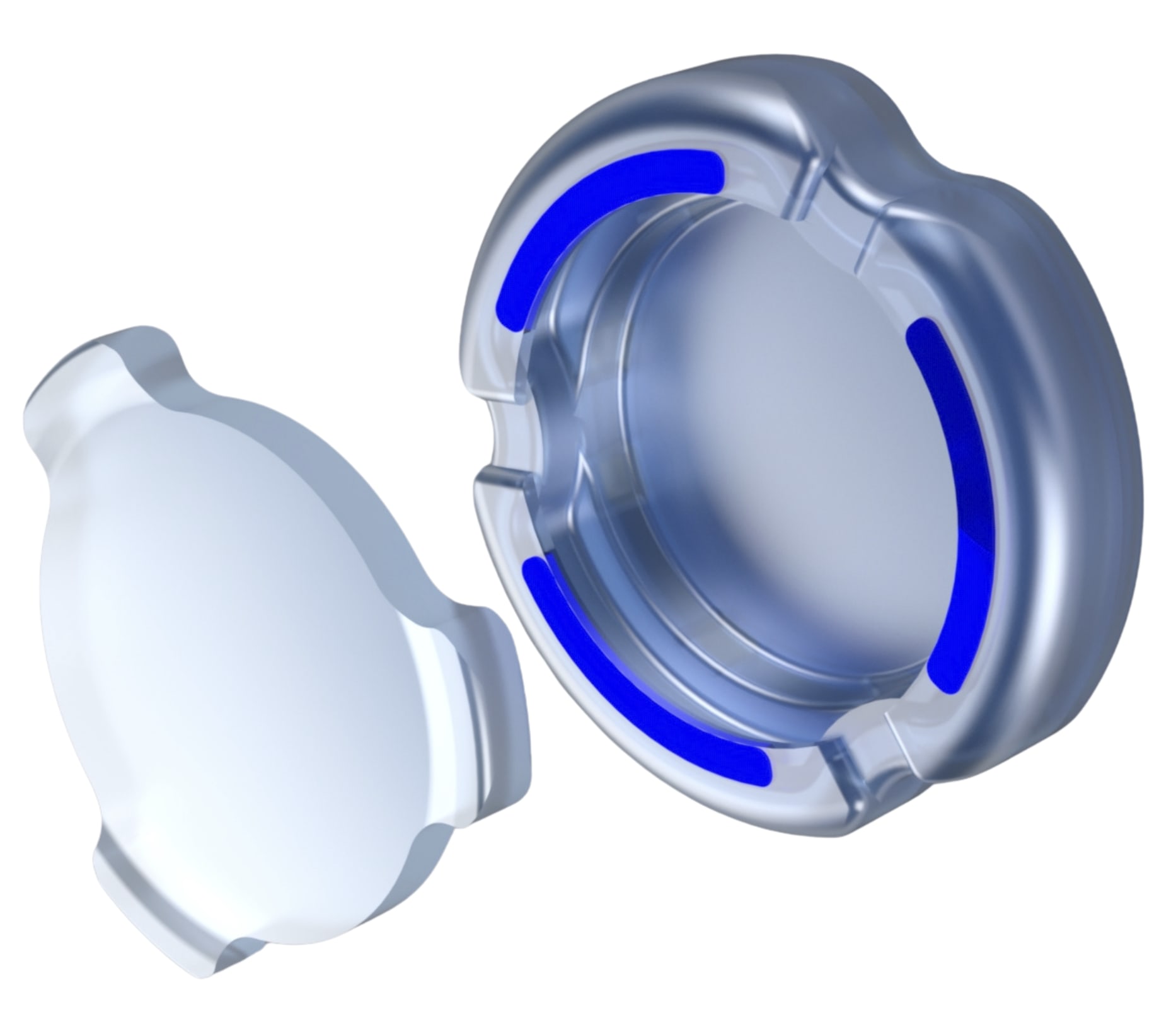
Atia Vision, Inc., a Shifamed portfolio company, has received Investigational Device Exemption (IDE) approval from the US Food and Drug Administration (FDA) to begin a traditional feasibility clinical study of its OmniVu Lens System. This novel intraocular lens (IOL) is designed to restore dynamic range of vision following cataract surgery, the company said in a press release.
According to Atia Vision, the OmniVu Lens System is comprised of 2 distinct components: a fluid-filled shape-changing base that provides focusing capability, or zoom function, and a front optic that docks into the base that provides the optical power. Additionally, engineered with a more physiologic shape to fill the capsular bag, the OmniVu Lens System is expected to preserve the eye's anatomic integrity and elasticity. This advanced design aims to provide patients with a more natural visual experience across all distances that continues to perform over time, the company said.
The OmniVu Lens System has been evaluated internationally in both first-in-human and feasibility clinical trials with over 75 lenses implanted to date and follow-up extending to 3 years. Clinical results thus far demonstrate continuous range of focus from far through near with 100% of patients achieving 20/20 or better uncorrected distance vision. Preliminary contrast sensitivity and patient-reported outcomes data demonstrate the potential for parity with monofocal lenses, the current standard for visual quality after cataract surgery, the company said in the press release.
"This IDE approval marks a pivotal milestone in our mission to transform the standard of care for cataract patients," said Mariam Maghribi, CEO of Atia Vision, in the press release. "OmniVu was developed to solve for the limitations of both accommodative and traditional lenses. Our technology represents a significant advancement in lens design by addressing both optical and functional compromises found in current solutions."