Oculis Holding AG announced positive topline results with OCS-05 in the Phase 2 ACUITY trial, which met the primary endpoint of safety and achieved statistical significance on several key efficacy-based secondary endpoints. The trial evaluated the safety, tolerability and efficacy of OCS-05, a neuroprotective candidate, in patients with acute optic neuritis, the company said in a press release.
The Phase 2 ACUITY (Acute OptiC NeUrITis of DemYelinating Origin) trial was a randomized, double-blind, placebo-controlled, multi-center trial, designed to evaluate OCS-05 (2 mg/kg/day or 3 mg/kg/day) administered intravenously once-daily for 5 days in patients with acute optic neuritis also receiving steroid. The study randomized 36 patients with recent onset (visual loss symptoms) of unilateral acute optic neuritis with a demyelinating origin, of which 33 patients received treatment and were included in the pre-specified modified intent-to-treat (mITT) analysis.
According to the company, positive results from the ACUITY trial showed that OCS-05 achieved the primary safety endpoint in addition to highlighting neuroprotective structural benefit and the ability to improve visual function in patients suffering from acute optic neuritis.
Primary endpoint was safety-based:
The percentage of patients with a shift from normal (baseline) to abnormal in electrocardiogram (ECG) parameters after study drug administration until Visit 4 (Day 15) was measured to evaluate cardiac safety. The results showed no difference in the percentage of patients with abnormal ECG parameters between the two treatment arms.
- Two patients in the OCS-05 arms (2 and 3 mg/kg/day) and one patient in the placebo arm had a shift from normal to abnormal in any ECG measures between baseline and Visit 4 (Day 15), both equivalent to 12.5%. Events observed in the OCS-05 arms were mild and transient and qualified as not clinically significant by central review reading center.
Secondary efficacy endpoints assessed changes in retinal structure:
Optical coherence tomography imaging was used to objectively measure the thickness of two different retinal segments in the affected eye to evaluate the potential neuroprotective effects of OCS-05 compared to placebo: 1) Ganglion Cell-Inner Plexiform Layer (GCIPL) and 2) Retinal Nerve Fiber Layer (RNFL). Results showed:
- A 43% improvement in GCIPL thickness mean change from baseline in favor of OCS-05 (3 mg/kg/day) + steroid compared to placebo + steroid at month 3 which was maintained through month 6 with p-values* of 0.049 and 0.052 at 3 and 6 months, respectively.
- A 28% improvement in RNFL thickness mean change from baseline in favor of OCS-05
(3 mg/kg/day) + steroid compared to placebo + steroid at month 3 reaching 30% improvement at month 6 with p-values* of 0.045 and 0.033 at 3 and 6 months, respectively.
Secondary efficacy endpoint assessed changes in visual function:
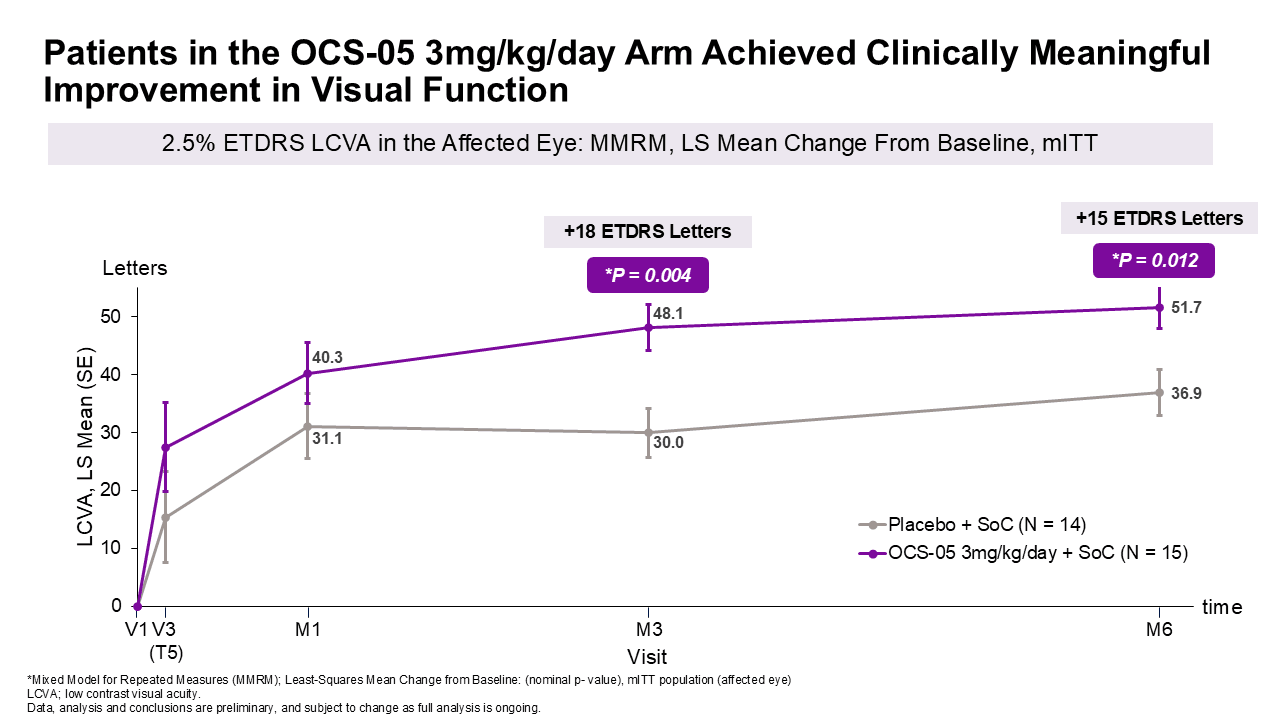
Changes in 2.5% ETDRS low-contrast letter acuity (LCVA) were measured to assess visual function improvement. Results showed:
- A favorable difference in LCVA mean change from baseline of approximately 18 letters at month 3 and approximately 15 letters at month 6 with OCS-05 (3 mg/kg/day) + steroid compared to placebo + steroid, with p-values^ of 0.004 and 0.012 at 3 and 6 months, respectively.
Treatment emergent adverse events (TEAEs):
- No drug-related serious adverse events (SAEs).
- No AEs leading to drug withdrawal or study discontinuation.
- Most frequently reported drug related AEs > 10% in the OCS-05 (2 or 3 mg/kg/day) + steroid treatment group were headache: two patients (10.5%), and acne: two patients (10.5%).
According to the company, OCS-05 has received orphan drug designation from the FDA and the European Medicines Agency for acute optic neuritis, a rare condition characterized by acute inflammation and demyelination of the optic nerve, often affecting young adults, in which retinal thinning is directly associated with vision loss and permanent visual impairment. There are currently no approved therapies specifically indicated for acute optic neuritis and despite steroids being used to treat inflammation and improve recovery, steroids are unable to provide neuroprotection to prevent vision loss.
In addition, the investigational new drug application for OCS-05 has also been cleared by the FDA, enabling the initiation of clinical development in the United States to support the global potential of OCS-05, the company said in the press release.