Thyroid eye disease (TED) has long been one of ophthalmology’s most challenging conditions to treat. Observed in approximately 50% of patients with Graves disease, TED affects women 5 times more frequently than men, with patients typically presenting in their 40s and exhibiting worse prognosis when diagnosed after age 50. Smoking is the strongest modifiable risk factor, whereas family history of autoimmune disorders and radioactive iodine therapy (especially in smokers) also contribute to disease risk and exacerbation.1 Patients suffer from debilitating symptoms, such as proptosis, diplopia, orbital pain, and—in severe cases—vision loss from optic nerve compression. For decades, our treatment arsenal was limited to corticosteroids for inflammation, supportive care, and surgical intervention for chronic complications. These approaches often provided only transient relief while carrying significant risks and side effects.
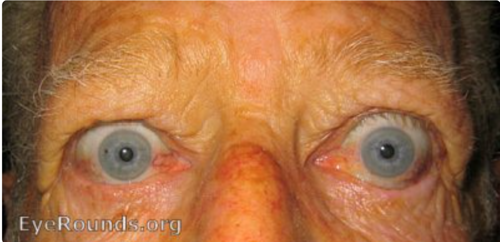
The landscape of TED treatment has been fundamentally transformed by advances in our understanding of disease pathophysiology, particularly the discovery of the insulin-like growth factor 1 receptor (IGF-1R) pathway as a key driver of pathogenesis. This mechanistic insight has enabled the development of targeted biologic therapies that address the underlying disease process rather than merely suppressing inflammation. Among these approaches, IGF-1R antagonism has emerged as the most transformative, offering the first truly disease-modifying treatment for TED.
Understanding TED Pathophysiology
Thyroid eye disease is an autoimmune condition most commonly associated with Graves disease, though it can occur in euthyroid or hypothyroid states.2-5 The disease affects approximately 50% of patients with Graves disease and demonstrates marked gender disparity, affecting women 5 times more frequently than men. Although TED can occur at any age, patients typically present in their 40s, with poorer prognosis in those diagnosed after age 50.
Central to TED pathogenesis is an intricate interaction between TSHR and IGF-1R on orbital fibroblasts and preadipocytes.6,7
The pathophysiology of TED centers on autoantibodies targeting the thyroid-stimulating hormone receptor (TSHR), which is overexpressed in orbital tissues. However, TSHR does not act alone. Central to TED pathogenesis is an intricate interaction between TSHR and IGF-1R on orbital fibroblasts and preadipocytes.6,7 TSHR forms a functional complex with IGF-1R through a process called transactivation—an established signaling mechanism between G protein-coupled receptors and receptor tyrosine kinases.
When this TSHR/IGF-1R complex is activated by autoantibodies, it initiates a cascade of pathologic events. IGF-1R, which is overexpressed in orbital fibroblasts, B cells, and T cells in TED patients, drives fibroblast proliferation, adipogenesis, proinflammatory cytokine production, and hyaluronan accumulation.4-8 These processes contribute to the characteristic tissue expansion, fibrosis, and chronic inflammation that define TED's clinical presentation.
Importantly, disrupting IGF-1R signaling blocks downstream effects of TSHR activation, including Erk pathway activation.7 This provides a “2 shots on goal” approach—interrupting pathogenic signaling from both TSHR and IGF-1R simultaneously. This mechanistic insight has positioned IGF-1R as the most promising therapeutic target in TED.
Limitations of Traditional Treatment
Traditional TED management has centered on corticosteroids, which remain recommended first-line treatment for moderate-to-severe TED in many parts of the world. Although corticosteroids effectively reduce acute orbital inflammation through broad immunosuppression, they show limited impact on proptosis and diplopia—the features most distressing to patients. Furthermore, they fail to address underlying autoimmune mechanisms or halt disease progression.
The limitations of corticosteroid therapy extend beyond efficacy. Relapse rates are high once steroids are tapered. Long-term use carries substantial risks, including hyperglycemia, hypertension, osteoporosis, psychiatric effects, and ocular complications, such as cataract progression and elevated intraocular pressure. These adverse effects often limit treatment duration and cumulative dose.
Supportive therapies, which may include artificial tears, selenium supplements, and eyelid taping, provide minimal relief. Surgical interventions such as orbital decompression, strabismus surgery, and eyelid retraction repair offer mechanical solutions but are typically reserved for the chronic, inactive phase of disease when tissue remodeling has stabilized.
IGF-1R Antagonism: A Mechanistic Breakthrough
Among emerging therapeutic approaches for TED, IGF-1R antagonism represents the most significant advance because it directly targets the core pathophysiology driving disease progression. IGF-1R controls cellular growth, differentiation, and survival, and its overexpression in orbital tissues creates the perfect storm for TED pathogenesis when combined with TSHR activation.6-7
By blocking IGF-1R, we can interrupt the entire pathogenic cascade at its source. This approach halts the signaling pathways responsible for B-cell and T-cell activation; fibrocyte and fibroblast proliferation; adipogenesis; and tissue expansion—all of which contribute to the clinical manifestations of TED. Critically, because of the functional crosstalk between TSHR and IGF-1R, blocking IGF-1R also prevents downstream signaling from TSHR activation, providing dual pathway inhibition from a single therapeutic intervention. 6-7
This mechanistic advantage distinguishes IGF-1R antagonism from other targeted approaches. Although interleukin-6 (IL-6) receptor antagonists (such as tocilizumab) can reduce inflammation and have shown benefit in some TED patients, they primarily affect downstream inflammatory cascades without addressing the fundamental driver of tissue remodeling. Similarly, neonatal Fc receptor (FcRn) antagonists work by reducing circulating autoantibody levels, but they may not adequately address cell-mediated pathogenesis. IGF-1R antagonism, by contrast, directly blocks the receptor complex responsible for orchestrating the disease process.
Teprotumumab: Proof of Concept for IGF-1R Targeting
Teprotumumab, a fully humanized monoclonal antibody targeting IGF-1R, has validated this therapeutic approach and established a new treatment paradigm. As the first FDA-approved medication specifically for TED, teprotumumab demonstrated that precise molecular targeting could achieve results previously thought impossible: sustained reduction in proptosis and reversal of chronic tissue changes.
The clinical evidence supporting IGF-1R antagonism is compelling. In a phase 2 study, 71% of teprotumumab-treated patients achieved at least 2 mm reduction in proptosis at 24 weeks compared to 20% receiving placebo.9 The phase 3 OPTIC trial demonstrated even more impressive results, with 83% of teprotumumab patients achieving the primary endpoint versus only 10% in the placebo group.10
Beyond proptosis reduction, teprotumumab demonstrated significant improvements across multiple clinical endpoints, including clinical activity score reduction (to 0 or 1), diplopia response, and quality of life measures. These benefits were achieved with a treatment regimen of 8 intravenous infusions over 21 weeks (10 mg/kg initial dose, then 20 mg/kg every 3 weeks).
The OPTIC-X trial provided important insights into IGF-1R antagonism’s efficacy across disease stages.11 This study enrolled patients who had received placebo in OPTIC, with a median disease duration of 12.9 months compared to 6.3 months in the original trial. Results demonstrated that patients with longer disease duration responded similarly to those treated earlier, suggesting that the IGF-1R pathway remains therapeutically accessible even in more established disease.
Perhaps most significantly, the phase 4 study evaluating teprotumumab in chronic TED with low disease activity established that IGF-1R antagonism provides benefit independent of disease duration or activity level.12
Perhaps most significantly, the phase 4 study evaluating teprotumumab in chronic TED with low disease activity established that IGF-1R antagonism provides benefit independent of disease duration or activity level.12 This led to FDA label expansion in 2023, approving teprotumumab for TED regardless of disease activity or duration—a validation of the approach's disease-modifying potential.
Orbital imaging studies have revealed that IGF-1R antagonism may reverse chronic pathologic changes in extraocular muscles and orbital fat. This represents a fundamental departure from traditional therapies that primarily target inflammatory symptoms without addressing the underlying tissue remodeling that causes persistent proptosis and motility disturbance.
Comparative Therapeutic Approaches
To appreciate the significance of IGF-1R antagonism, it’s helpful to briefly consider other targeted approaches that have been explored in TED:
IL-6 receptor antagonists, such as tocilizumab, reduce orbital inflammation by blocking IL-6 signaling, which promotes T helper 17 cell differentiation and proinflammatory cytokine production. Although these agents can reduce disease activity scores in some patients, their effects on proptosis and diplopia are modest. They address downstream inflammation but miss the upstream receptor signaling that drives tissue expansion.
FcRn antagonists work by preventing immunoglobulin G recycling, thereby reducing levels of pathogenic autoantibodies, including those targeting TSHR. Although it is conceptually appealing for antibody-mediated diseases, this approach has limitations in TED. Some patients present with severe disease despite undetectable anti-TSHR antibodies, suggesting important roles for cell-mediated immunity. Additionally, FcRn inhibition affects all immunoglobulin G antibodies, not just pathogenic ones, potentially compromising normal immune function.
TSHR antagonists are under development based on TSHR’s central role in disease initiation. Although TSHR is clearly important in TED pathogenesis, its relative anatomic restriction compared to IGF-1R's broader expression does not necessarily translate to therapeutic advantage. Recent findings suggest TSHR may be more widely expressed than previously thought,13-14 and its functional coupling with IGF-1R means that blocking IGF-1R effectively inhibits TSHR-mediated signaling.
What distinguishes IGF-1R antagonism is its ability to block both IGF-1R and TSHR pathogenic signaling through their functional interaction while directly targeting the receptor overexpressed on the key pathogenic cell types—orbital fibroblasts, B cells, and T cells.4-5 This provides more comprehensive disease control than approaches targeting downstream effects or single pathways.
Safety Profile and Clinical Management
Clinical trial experience with teprotumumab has established the safety profile of IGF-1R antagonism in TED.9-10 Common adverse events include muscle spasm, nausea, alopecia, diarrhea, fatigue, hyperglycemia, hearing impairment, dysgeusia, headache, and dry skin. Less common, but more serious, reactions include hearing loss, Hashimoto encephalopathy, and exacerbation of preexisting inflammatory bowel disease.
The majority of adverse events were mild to moderate in severity and manageable, with few treatment discontinuations. This safety profile compares favorably to long-term corticosteroid therapy, which carries risks of hypertension, diabetes, osteoporosis, psychiatric effects, and opportunistic infections.
Notably, IGF-1R is ubiquitously expressed throughout the body, raising theoretical concerns about on-target effects in nonorbital tissues. However, clinical experience has demonstrated that these effects are manageable, and the benefits in moderate-to-severe TED clearly outweigh the risks for appropriate patients. Ongoing postmarketing surveillance continues to characterize the long-term safety profile.
For ophthalmologists implementing IGF-1R antagonist therapy, patient selection should account for disease activity, duration, severity, and individual risk factors. Pretreatment evaluation should include comprehensive thyroid function assessment with appropriate management of hyperthyroidism. Audiometric baseline testing enables monitoring for hearing changes during treatment. Patients with inflammatory bowel disease require careful consideration given the risk of disease exacerbation.
Future of IGF-1R Antagonism
The success of teprotumumab as the first IGF-1R antagonist for TED has catalyzed development of next-generation agents designed to optimize the benefit-risk profile while maintaining the mechanistic advantages of IGF-1R targeting. Key innovations under development include the following:
-
Enhanced molecular designs that may improve target specificity and tissue distribution, potentially minimizing systemic exposure while maintaining efficacy in orbital tissues. Some approaches use antibody engineering to modulate pharmacokinetic properties.
-
Optimized dosing regimens with fewer and shorter infusions to reduce treatment burden. Investigational IGF-1R antagonists in clinical development aim to achieve therapeutic effect with as few as 4 infusions over shorter total treatment duration compared to teprotumumab's 8-infusion regimen.
-
Half-life extension technology enabling less frequent dosing intervals. By extending the time between doses, these approaches could improve convenience and adherence while reducing the cumulative time patients spend receiving infusions.
-
Novel delivery systems including subcutaneous formulations with auto-injector technology represent perhaps the most transformative innovation. This could enable home-based self-administration, eliminating the need for infusion center visits. Such convenience would dramatically improve access and reduce healthcare costs while maintaining the mechanistic benefits of IGF-1R targeting.
These innovations demonstrate the maturation of IGF-1R antagonism as a therapeutic approach. The initial proof-of-concept with teprotumumab has been validated, and the field is now advancing toward more refined implementations of the same fundamental mechanism.
Clinical Implementation for Ophthalmologists
For comprehensive ophthalmologists encountering TED in practice, understanding IGF-1R antagonism has become essential. Many patients will present having read about teprotumumab or will have been referred specifically for evaluation of treatment candidacy. Being able to discuss the mechanism, expected outcomes, treatment course, and potential side effects is increasingly important.
Key points for patient discussions include:
-
IGF-1R antagonism represents targeted therapy that addresses the underlying disease mechanism, not just symptoms.
-
Treatment involves a series of infusions over several months with regular monitoring.
-
Expected benefits include proptosis reduction, improvement in diplopia, and reduction in inflammatory signs.
-
Most patients experience some side effects, but these are generally manageable.
-
The approach has been proven effective across disease stages, from active inflammatory disease to chronic, previously “burnt out” cases.
For ophthalmologists comanaging these patients with endocrinology or oculoplastic colleagues, monitoring for ocular complications remains important. Although IGF-1R antagonism can dramatically improve TED manifestations, patients may still require management of exposure keratopathy; monitoring for optic neuropathy; or eventual surgical correction of residual strabismus or eyelid malposition.
Broader Implications
The success of IGF-1R antagonism in TED has implications beyond this single disease. The identification of receptor crosstalk between TSHR and IGF-1R as a pathogenic mechanism, and the demonstration that blocking one receptor can interrupt signaling from both, provides a model for understanding and treating other autoimmune conditions.
This approach exemplifies how detailed mechanistic understanding can enable development of precisely targeted therapies with superior efficacy and safety profiles compared to broad immunosuppression. The strategy of targeting receptor tyrosine kinases involved in pathogenic signaling cascades may prove applicable to other immune-mediated ophthalmic diseases.
For ophthalmology as a specialty, TED’s transformation through IGF-1R antagonism foreshadows similar advances in other conditions. We are entering an era where molecular understanding of disease mechanisms guides therapeutic development, enabling precision medicine approaches tailored to specific pathogenic pathways.
Conclusions
IGF-1R antagonism represents a fundamental breakthrough in TED treatment, transforming the disease from one with limited medical options to one with highly effective targeted therapy. By directly blocking the receptor complex central to disease pathogenesis, this approach achieves sustained improvements in proptosis, diplopia, inflammation, and quality of life that were previously unattainable with conventional treatments.
Teprotumumab, as the first IGF-1R antagonist approved for TED, has validated this therapeutic strategy and established proof of concept. The robust clinical evidence—demonstrating efficacy across disease stages, durations, and activity levels—confirms that IGF-1R represents the optimal therapeutic target in TED. The ability to interrupt both IGF-1R and TSHR pathogenic signaling through a single intervention, while directly targeting the key pathogenic cell types, provides mechanistic advantages over alternative approaches.
As next-generation IGF-1R antagonists advance through clinical development, we anticipate further improvements in convenience, tolerability, and accessibility while maintaining the core mechanistic benefits. The evolution toward subcutaneous self-administration represents a particularly transformative advance that could dramatically expand treatment access.
For ophthalmologists, understanding IGF-1R antagonism and its role in TED management has become essential. Whether ophthalmologists are managing these patients directly or comanaging with subspecialists, their ability to discuss this therapeutic approach and its implications is increasingly important as more patients seek or receive this treatment.
The journey from discovering IGF-1R’s role in TED pathogenesis to FDA-approved targeted therapy demonstrates the power of translational research to transform patient care. As we continue to refine IGF-1R antagonist approaches and develop increasingly sophisticated therapeutic options, patients with TED can look forward to a future with more effective, convenient, and accessible treatment that addresses the fundamental cause of their disease rather than merely managing symptoms. OM
References
1. Bartalena L, Piantanida E, Gallo D, et al. Epidemiology, natural history, risk factors, and prevention of Graves' orbitopathy. Front Endocrinol. 2020;11:615993.
2. Gerding MN, van der Meer JW, Broenink M, et al. Association of thyrotrophin receptor antibodies with the clinical features of Graves' ophthalmopathy. Clin Endocrinol (Oxf). 2000;52(3):267-271.
3. Ludgate M, Crisp M, Lane C, et al. The thyrotropin receptor in thyroid eye disease. Thyroid. 1998;8(5):411-413.
4. Pritchard J, Han R, Horst N, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin-like growth factor I receptor pathway. J Immunol. 2003;170(12):6348-6354.
5. Pritchard J, Horst N, Cruikshank W, et al. Igs from patients with Graves' disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168(2):942-950.
6. Krieger CC, Neumann S, Place RF, et al. Bidirectional TSH and IGF-1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves' disease immunoglobins. J Clin Endocrinol Metab. 2015;100(3):1071-1077.
7. Tsui S, Naik V, Hoa N, et al. Evidence for an association between thyroid-stimulating hormone and insulin-like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol. 2008;181(6):4397-4405.
8. Woeller CF, Roztocil E, Hammond C, et al. TSHR signaling stimulates proliferation through PI3K/Akt and induction of miR-146a and miR-155 in thyroid eye disease orbital fibroblasts. Invest Ophthalmol Vis Sci. 2019;60(13):4336-4345.
9. Smith TJ, Kahaly GJ, Ezra DG, et al. Teprotumumab for thyroid-associated ophthalmopathy. N Engl J Med. 2017;376(18):1748-1761.
10. Douglas RS, Kahaly GJ, Patel A, et al. Teprotumumab for the treatment of active thyroid eye disease. N Engl J Med. 2020;382(4):341-352.
11. Douglas RS, Kahaly GJ, Ugradar S, et al. Teprotumumab efficacy, safety, and durability in longer-duration thyroid eye disease and re-treatment: OPTIC-X study. Ophthalmology. 2022;129(4):438-449.
12. Kahaly GJ, Douglas RS, Engel K, et al. Teprotumumab for chronic thyroid eye disease. N Engl J Med. 2024;390(20):1879-1888.
13. Wakelkamp IM, Bakker O, Baldeschi L, et al. TSH-R expression and cytokine profile in orbital tissue of active vs. inactive Graves' ophthalmopathy patients. Clin Endocrinol (Oxf). 2003;58(3):280-287.
14. Bahn RS, Dutton CM, Joba W, et al. Thyrotropin receptor expression in cultured Graves orbital preadipocyte fibroblasts is stimulated by thyrotropin. Thyroid. 1998;8(2):193-196.









